FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 31 março 2025

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.

Messages from the COVID-19 Response Task Force
FDA Open Meeting on Moderna COVID-19 Vaccine, Part 2

FDA Revokes Emergency Use Authorization for Monoclonal

Biomedicines, Free Full-Text

Gov. John Bel Edwards - These safe and effective COVID vaccines
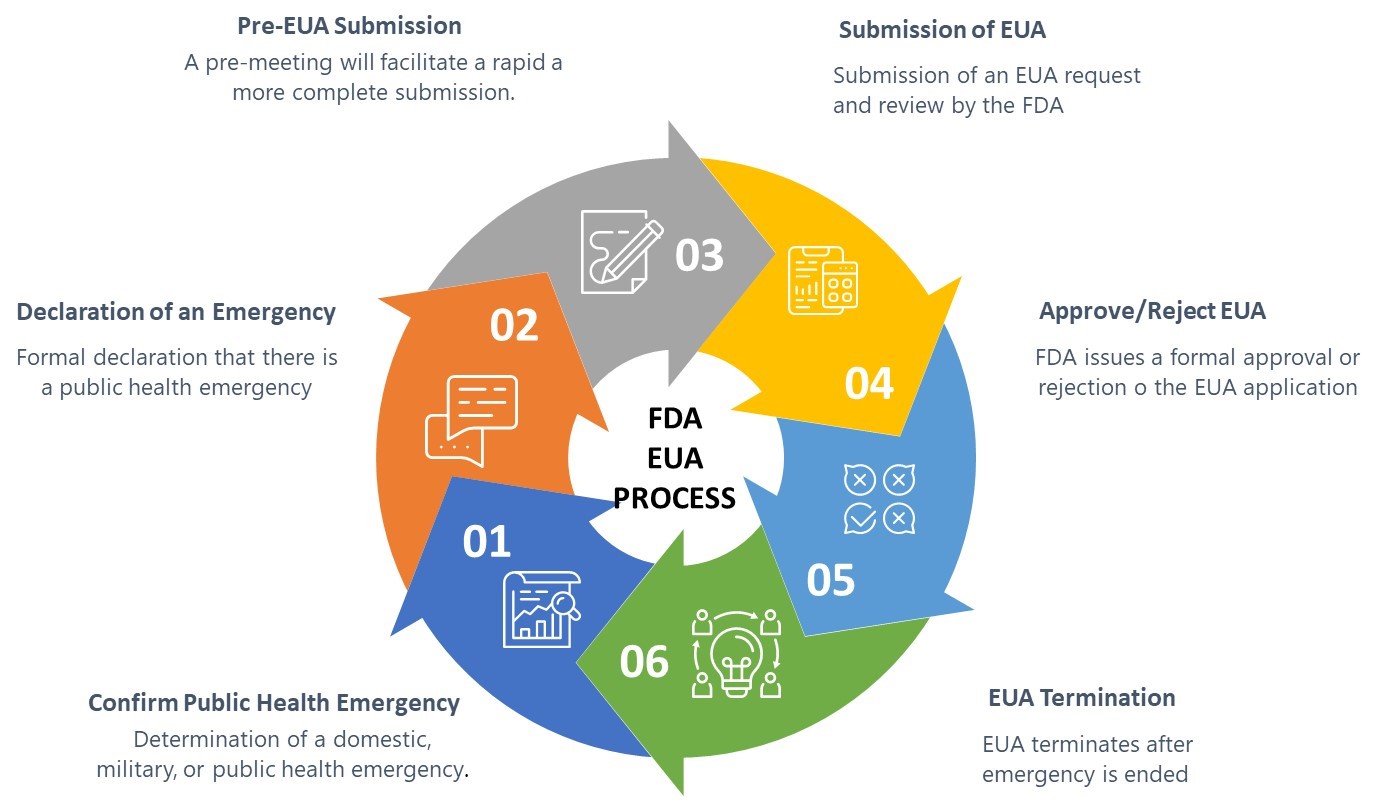
Navigating The FDA's Emergency Use Authorization Process
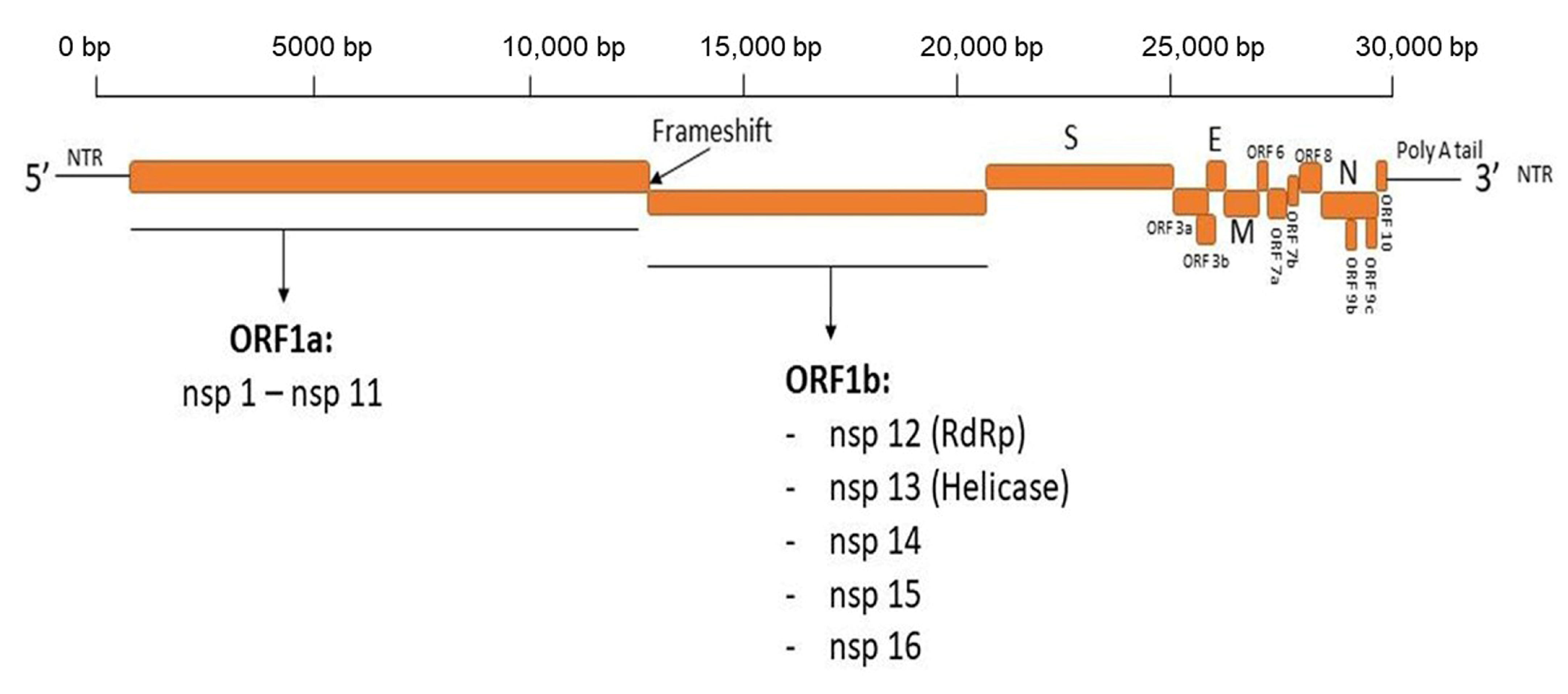
Antibodies, Free Full-Text

Federal Register :: Authorizations of Emergency Use of Certain
FDA Open Meeting on Johnson & Johnson COVID-19 Vaccine, Part 5
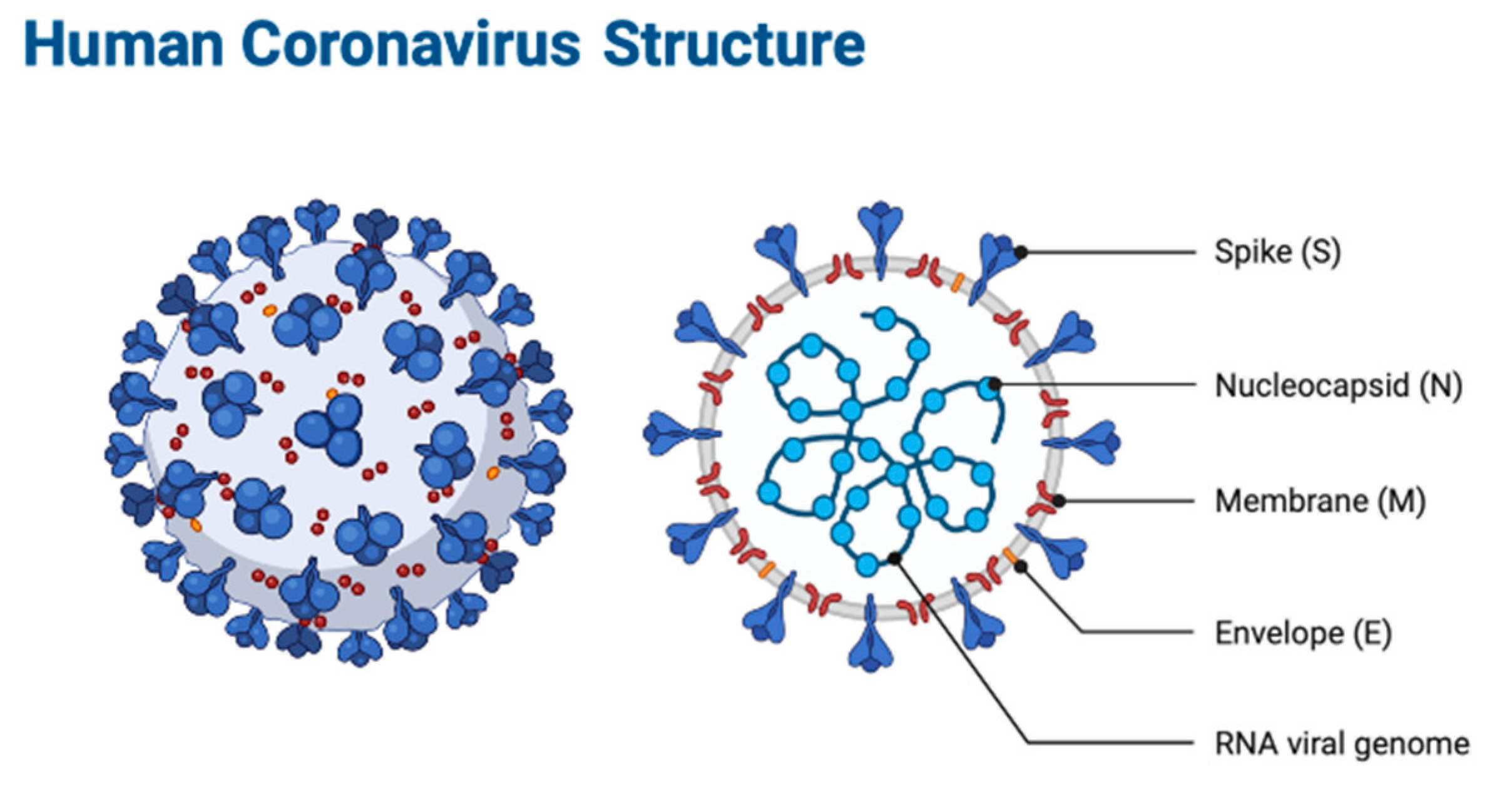
IJMS, Free Full-Text
Recomendado para você
-
 See Who Pledged Jewish Future Pledge31 março 2025
See Who Pledged Jewish Future Pledge31 março 2025 -
 Isaac Azar, MD, Aventura, FL Emergency Medicine Physician31 março 2025
Isaac Azar, MD, Aventura, FL Emergency Medicine Physician31 março 2025 -
Congratulations to - St. Vincent Charity Medical Center31 março 2025
-
 Doctors - Brighton Family & Women's Clinic31 março 2025
Doctors - Brighton Family & Women's Clinic31 março 2025 -
 Behdad David Besharatian, MD profile31 março 2025
Behdad David Besharatian, MD profile31 março 2025 -
Health Care Policy Priorities for 117th Congress31 março 2025
-
 Ecumenical Council of Hungarian Churches concludes its visit to Palestine - Daily News Hungary31 março 2025
Ecumenical Council of Hungarian Churches concludes its visit to Palestine - Daily News Hungary31 março 2025 -
 Newsletter January 2022: Editorial – Is anaesthesia mortality a source of stress for the anaesthesiologist?31 março 2025
Newsletter January 2022: Editorial – Is anaesthesia mortality a source of stress for the anaesthesiologist?31 março 2025 -
 A Letter to Our Patients on Racism, by docs4blm31 março 2025
A Letter to Our Patients on Racism, by docs4blm31 março 2025 -
Ocular Immunology and Inflammation: Vol 31, No 631 março 2025
você pode gostar
-
 JNayaDS — Fnaf-2-Boys 25.6.2020 #clipstudiopaint +31 março 2025
JNayaDS — Fnaf-2-Boys 25.6.2020 #clipstudiopaint +31 março 2025 -
 JOHN WICK 4 Final Trailer Brasileiro Legendado (2023)31 março 2025
JOHN WICK 4 Final Trailer Brasileiro Legendado (2023)31 março 2025 -
 the sims freeplay dinheiro infinito nuvem adiantada31 março 2025
the sims freeplay dinheiro infinito nuvem adiantada31 março 2025 -
 Epomaker Mystery Box31 março 2025
Epomaker Mystery Box31 março 2025 -
 Leagues Cup 2023: Se definieron los grupos del torneo para los31 março 2025
Leagues Cup 2023: Se definieron los grupos del torneo para los31 março 2025 -
 Assistir Slime Taoshite 300-nen, Shiranai Uchi ni Level Max ni31 março 2025
Assistir Slime Taoshite 300-nen, Shiranai Uchi ni Level Max ni31 março 2025 -
 Faqueiro Cobre Rose Gold 24 Pçs Inox - Talheres Garfo Faca Colher e Sobremesa - Presentes Criativos e Diferentes - L3 Store31 março 2025
Faqueiro Cobre Rose Gold 24 Pçs Inox - Talheres Garfo Faca Colher e Sobremesa - Presentes Criativos e Diferentes - L3 Store31 março 2025 -
 How To Make Money FAST In Driving Simulator!31 março 2025
How To Make Money FAST In Driving Simulator!31 março 2025 -
 Fazer convite online convite digital Barbie em Paris31 março 2025
Fazer convite online convite digital Barbie em Paris31 março 2025 -
 The Last of Us' PC port is bad, but the bugs are great31 março 2025
The Last of Us' PC port is bad, but the bugs are great31 março 2025
