New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect
Por um escritor misterioso
Last updated 30 março 2025


Douglas Wolf on LinkedIn: #usepa #syngenta #oecd #iata #usepa #syngentaproud #science

New approach methodologies in human regulatory toxicology – Not if, but how and when! - ScienceDirect

Sustainability, Free Full-Text

Regulatory assessment of chemical mixtures: Requirements, current approaches and future perspectives - ScienceDirect

Transgenic Mouse Models Transferred into the Test Tube: New Perspectives for Developmental Toxicity Testing In Vitro?: Trends in Pharmacological Sciences

A Call For Action On The Development and Implementation o - 2021 - Regulatory To, PDF, Toxicology

Toxicity testing: creating a revolution based on new technologies: Trends in Biotechnology
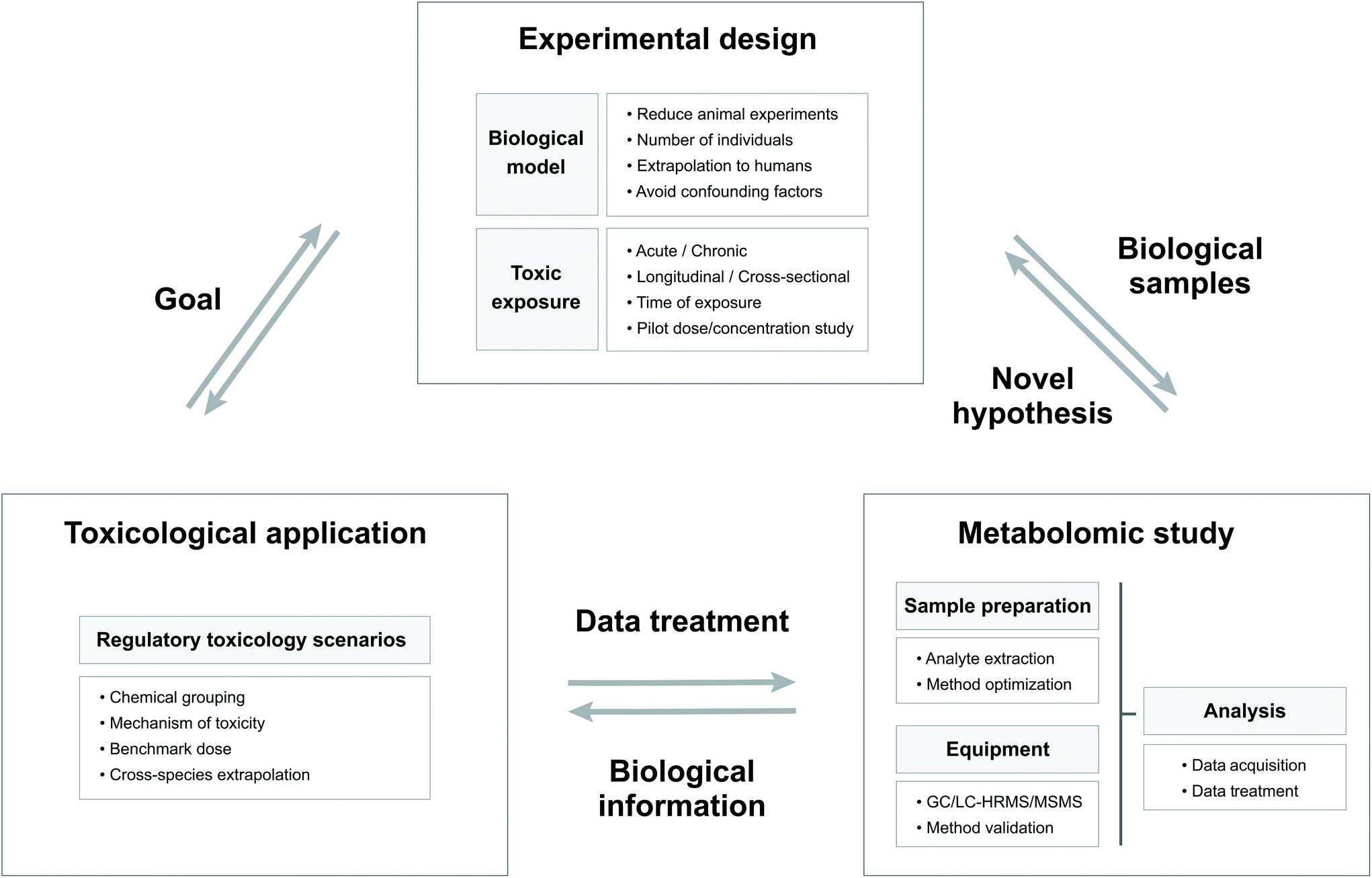
Approaches in metabolomics for regulatory toxicology applications - Analyst (RSC Publishing) DOI:10.1039/D0AN02212H

New Approach Methodologies (NAMs) for safety testing of complex food matrices: A review of status, considerations, and regulatory adoption - ScienceDirect

Assessing Toxicity with Human Cell-Based In Vitro Methods: Trends in Molecular Medicine
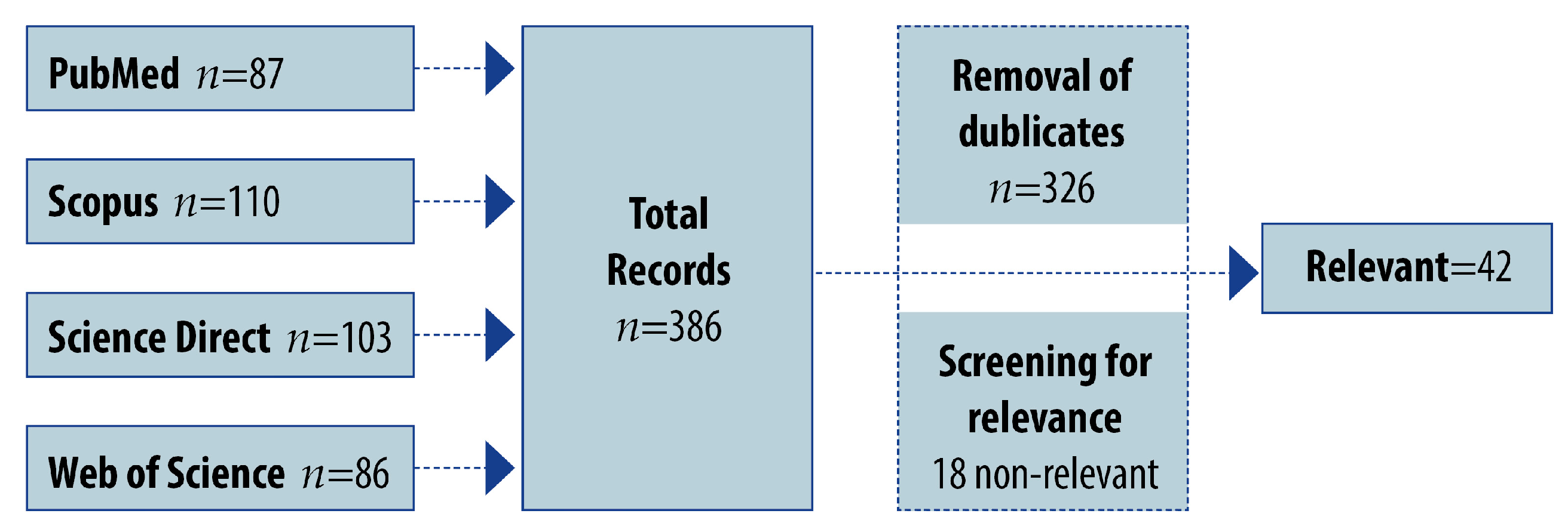
Pharmaceutics, Free Full-Text

Innovation in regulatory approaches for endocrine disrupting chemicals: The journey to risk assessment modernization in Canada - ScienceDirect

Application of a new approach method (NAM) for inhalation risk assessment - ScienceDirect

Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy

New approach methodologies (NAMs) for human-relevant biokinetics predictions
Recomendado para você
-
 Press Archive - Stanford Law School30 março 2025
Press Archive - Stanford Law School30 março 2025 -
kelly godoy (@GodoySexy) / X30 março 2025
-
 Vani Hari - Wikipedia30 março 2025
Vani Hari - Wikipedia30 março 2025 -
 Iñaki Godoy: Age, height and facts about One Piece live-action Luffy - PopBuzz30 março 2025
Iñaki Godoy: Age, height and facts about One Piece live-action Luffy - PopBuzz30 março 2025 -
 Season 4, Ceazar Chan Wiki30 março 2025
Season 4, Ceazar Chan Wiki30 março 2025 -
Fishing and integrated subsistence in central Mexican domesticated30 março 2025
-
 We Have Been There Before – American University of Health Sciences30 março 2025
We Have Been There Before – American University of Health Sciences30 março 2025 -
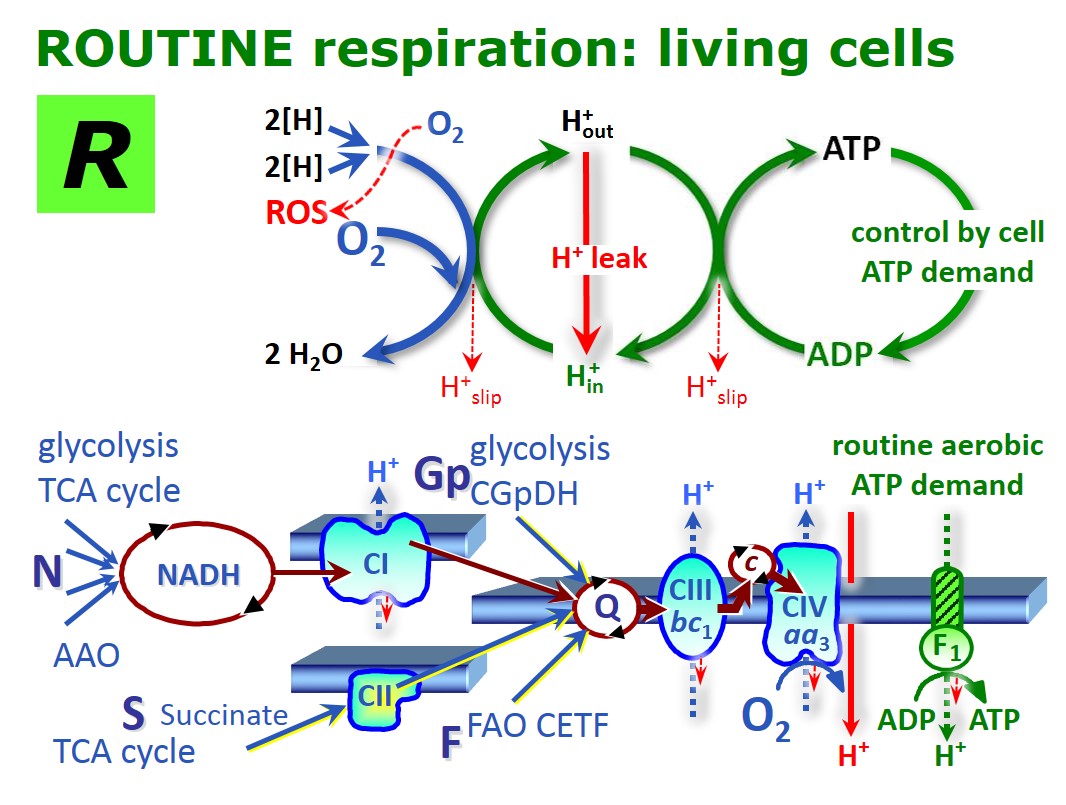 ROUTINE respiration - Bioblast30 março 2025
ROUTINE respiration - Bioblast30 março 2025 -
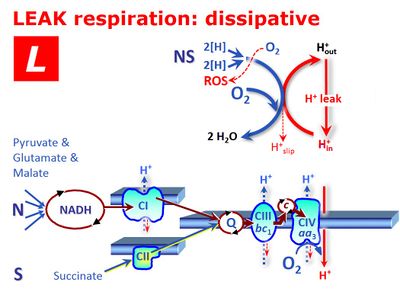 LEAK respiration - Bioblast30 março 2025
LEAK respiration - Bioblast30 março 2025 -
 Guest essay: Comments and questions on NU's proposed stadium and zoning change - Evanston RoundTable30 março 2025
Guest essay: Comments and questions on NU's proposed stadium and zoning change - Evanston RoundTable30 março 2025
você pode gostar
-
 How Many Stocks Should I Own? Portfolio Diversification Guide (2023)30 março 2025
How Many Stocks Should I Own? Portfolio Diversification Guide (2023)30 março 2025 -
 G1 > Carros - NOTÍCIAS - GM lança Chevrolet Agile e inaugura nova família de carros30 março 2025
G1 > Carros - NOTÍCIAS - GM lança Chevrolet Agile e inaugura nova família de carros30 março 2025 -
 Love cats flat square icon with long shadows. #love #loveillustration #flaticons #vectoricons #flatdesign30 março 2025
Love cats flat square icon with long shadows. #love #loveillustration #flaticons #vectoricons #flatdesign30 março 2025 -
 Pocket Bravery: promissor jogo de luta 2D brasileiro busca30 março 2025
Pocket Bravery: promissor jogo de luta 2D brasileiro busca30 março 2025 -
 Relógio Apple Watch Ultra 49MM 4G (GPS/ CELULAR) - BRS30 março 2025
Relógio Apple Watch Ultra 49MM 4G (GPS/ CELULAR) - BRS30 março 2025 -
 Kit Festa Gata Marie 113 peças (10 pessoas) painel e cx - Ateliê30 março 2025
Kit Festa Gata Marie 113 peças (10 pessoas) painel e cx - Ateliê30 março 2025 -
 Cool Boarders 4 Motocross Test Drive 6 Tour Racing Playstation 1 2 PS1 PS2 Games30 março 2025
Cool Boarders 4 Motocross Test Drive 6 Tour Racing Playstation 1 2 PS1 PS2 Games30 março 2025 -
 The best team to beat Red in Pokemon Gold and Silver30 março 2025
The best team to beat Red in Pokemon Gold and Silver30 março 2025 -
 Sonic 2' é exploração enfadonha do universo do personagem - 07/0430 março 2025
Sonic 2' é exploração enfadonha do universo do personagem - 07/0430 março 2025 -
 How are chess pieces related to family? - Quora30 março 2025
How are chess pieces related to family? - Quora30 março 2025
