GHGH Formula - C14H26O11 - Over 100 million chemical compounds
Por um escritor misterioso
Last updated 30 março 2025

GHGH contains total 51 atom(s); 26 Hydrogen atom(s), 14 Carbon atom(s), and 11 Oxygen atom(s). Learn more about GHGH chemical formula at Mol-Instincts.

degradation of long- chain hydrocarbon compounds
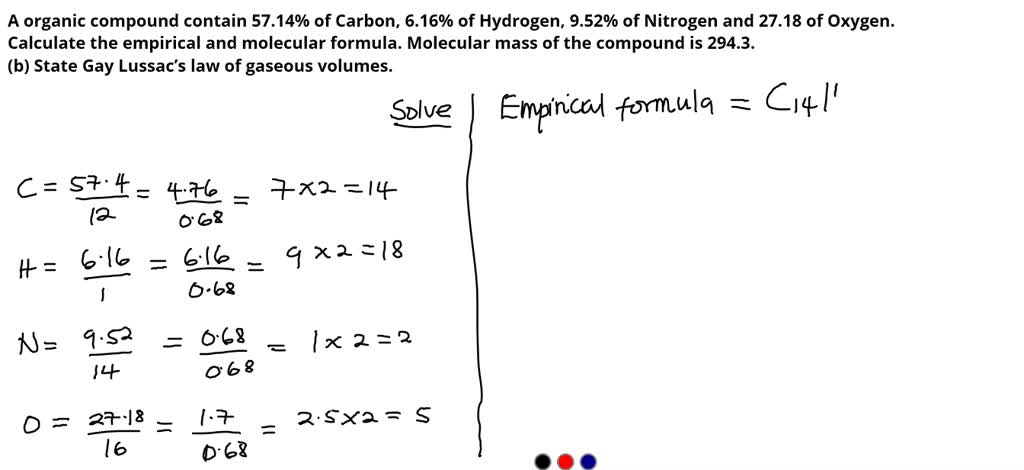
SOLVED: A organic compound contain 57.14% of Carbon, 6.16% of Hydrogen, 9.52% of Nitrogen and 27.18 of Oxygen. Calculate the empirical and molecular formula. Molecular mass of the compound is 294.3. -3-(b)
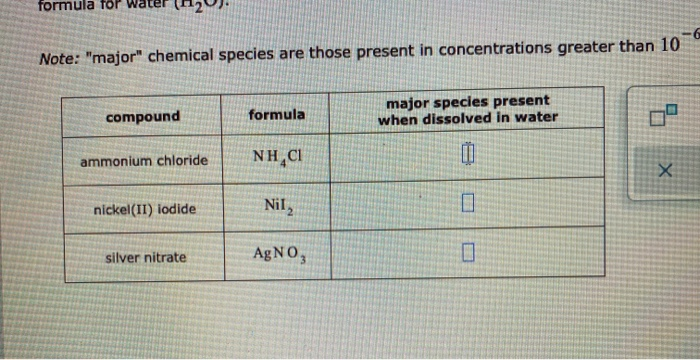
Solved IULUI IUL WUL - Note: major chemical species are
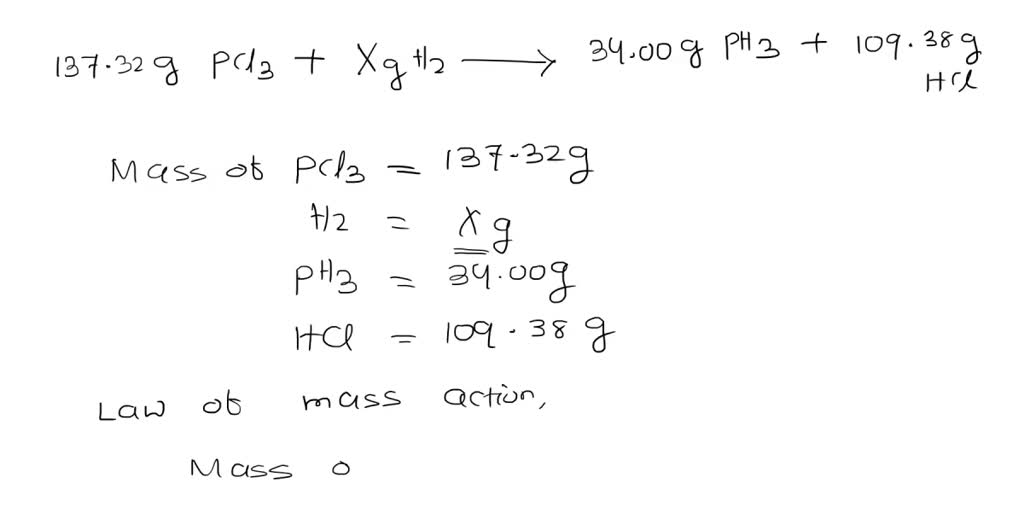
SOLVED: Determine the value of X in the following otherwise correct description of a chemical reaction: (137.32 g PCl3) + (X g H2) + (34.00 g PH3) = (109.38 g HCl) A
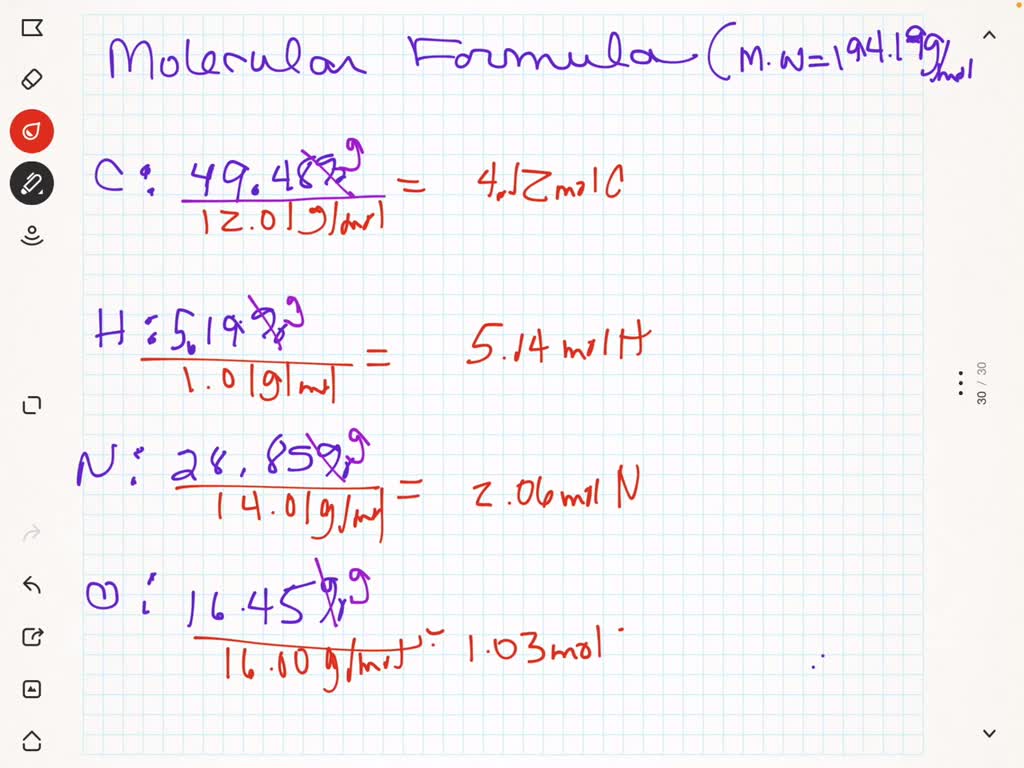
SOLVED: Determine the molecular formula of a compound that is 49.48% carbon, 5.19% hydrogen, 28.85% nitrogen, and 16.48% oxygen. The molecular weight is 194.19 g/mol. C8H12N4O2

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g

4-Heptanone SDF/Mol File - C7H14O - Over 100 million chemical compounds
Solved 11. A compound has the composition of 66.6% Carbon

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
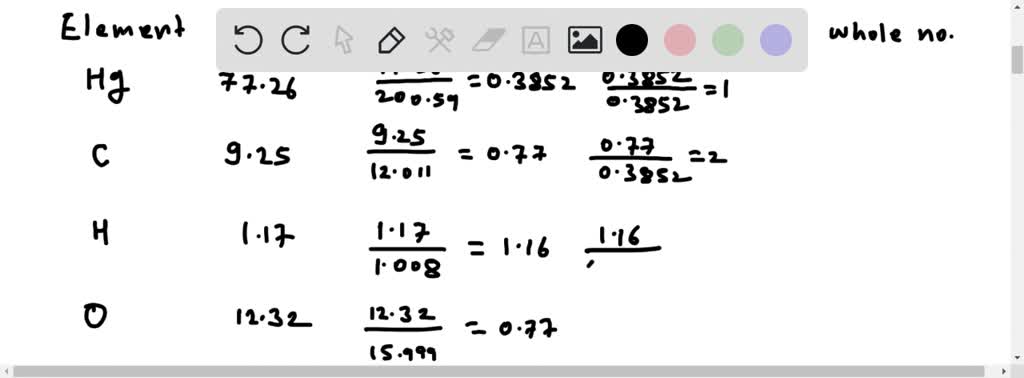
⏩SOLVED:One compound of mercury with a molar mass of 519 contains…

SOLVED: What is the formula for a solid compound that contains 42.11% C, 51.46% O, and 6.43% H and having a molecular weight of about 341 (molecular weight of O-16g/mole, H-1g/mole, C-12g/mole): (

A compound with a known molecular weight (146.99 g/mol) that contains only C, H, and Cl was studied by combustion analysis. When a 0.367g sample was combusted, 0.659g of CO2 and 0.0892g
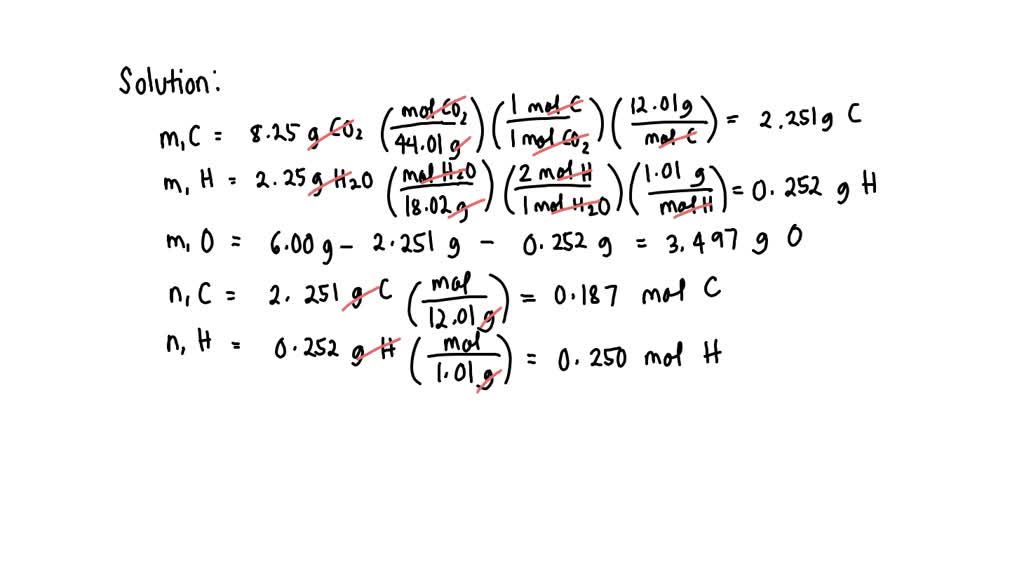
SOLVED: 6.00g of a certain Compound X, known to be made of carbon, hydrogen, and perhaps oxygen, and to have a molecular molar mass of 192 g/mol, is burned completely in excess
Recomendado para você
-
Steam Workshop::ghgh30 março 2025
-
 Stream Emőke Ambrus Listen to ghgh playlist online for free on30 março 2025
Stream Emőke Ambrus Listen to ghgh playlist online for free on30 março 2025 -
 GHGH Summit for young girls across Monterey County30 março 2025
GHGH Summit for young girls across Monterey County30 março 2025 -
 ghgh Minecraft Skins30 março 2025
ghgh Minecraft Skins30 março 2025 -
 GHGH by Tiverton Perfumed Shower Gel For Women 5.1 oz * New30 março 2025
GHGH by Tiverton Perfumed Shower Gel For Women 5.1 oz * New30 março 2025 -
Ghgh - song and lyrics by Chris Espo, Cortez30 março 2025
-
 ghgh - Python Package Health Analysis30 março 2025
ghgh - Python Package Health Analysis30 março 2025 -
ghgh Nova Skin30 março 2025
-
 ghgh - Images30 março 2025
ghgh - Images30 março 2025 -
 Real Talk ( G.H.G.H Music)30 março 2025
Real Talk ( G.H.G.H Music)30 março 2025
você pode gostar
-
 Name Tag Border Clipart Transparent Background, Name Border, Border, Gaming, Name PNG Image For Free Download30 março 2025
Name Tag Border Clipart Transparent Background, Name Border, Border, Gaming, Name PNG Image For Free Download30 março 2025 -
Mario Kart tournament this - Raymore Parks and Recreation30 março 2025
-
UK Anime Network30 março 2025
-
 ESCOLA DE XADREZ Livraria Martins Fontes Paulista30 março 2025
ESCOLA DE XADREZ Livraria Martins Fontes Paulista30 março 2025 -
 Scary Games Online (FREE)30 março 2025
Scary Games Online (FREE)30 março 2025 -
 Location Icon Gif Transparent Transparent PNG - 860x1099 - Free Download on NicePNG30 março 2025
Location Icon Gif Transparent Transparent PNG - 860x1099 - Free Download on NicePNG30 março 2025 -
 Nightmare Fredbear Fnaf coloring pages, Fnaf drawings, Nightmare30 março 2025
Nightmare Fredbear Fnaf coloring pages, Fnaf drawings, Nightmare30 março 2025 -
Apps Android no Google Play: OLX Portugal SA30 março 2025
-
 1998 Hot Wheels Surf 'N Fun Series 2/4 VW Bug Blue Volkswagen Beetle #962 NOC30 março 2025
1998 Hot Wheels Surf 'N Fun Series 2/4 VW Bug Blue Volkswagen Beetle #962 NOC30 março 2025 -
 40 Best Shows on HBO Max - TV Series to Watch on HBO Max in 202330 março 2025
40 Best Shows on HBO Max - TV Series to Watch on HBO Max in 202330 março 2025



