FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 21 março 2025

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

agitation that may happen with dementia due to Alzheimer's disease Questionnaire
Video: Using the REXULTI Savings Card
Vistgen And Relmada: Competitors In Depression Treatment (NASDAQ:RLMD)

Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America
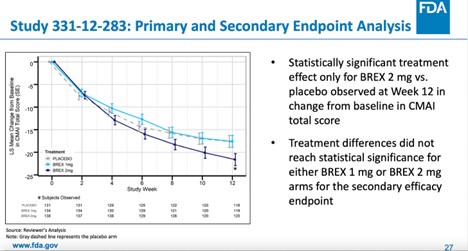
Not Everyone Agreed With FDA Approval of Antipsychotic Rexulti for Agitation - Mad In America

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services

Rexulti, the first drug to relieve Alzheimer's emotions - TimesKuwait
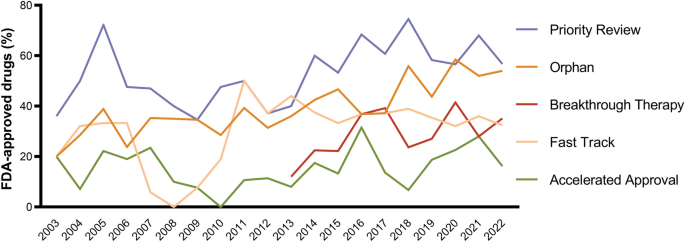
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy

FDA's accelerated drug approvals often lack confirmatory evidence : Shots - Health News : NPR
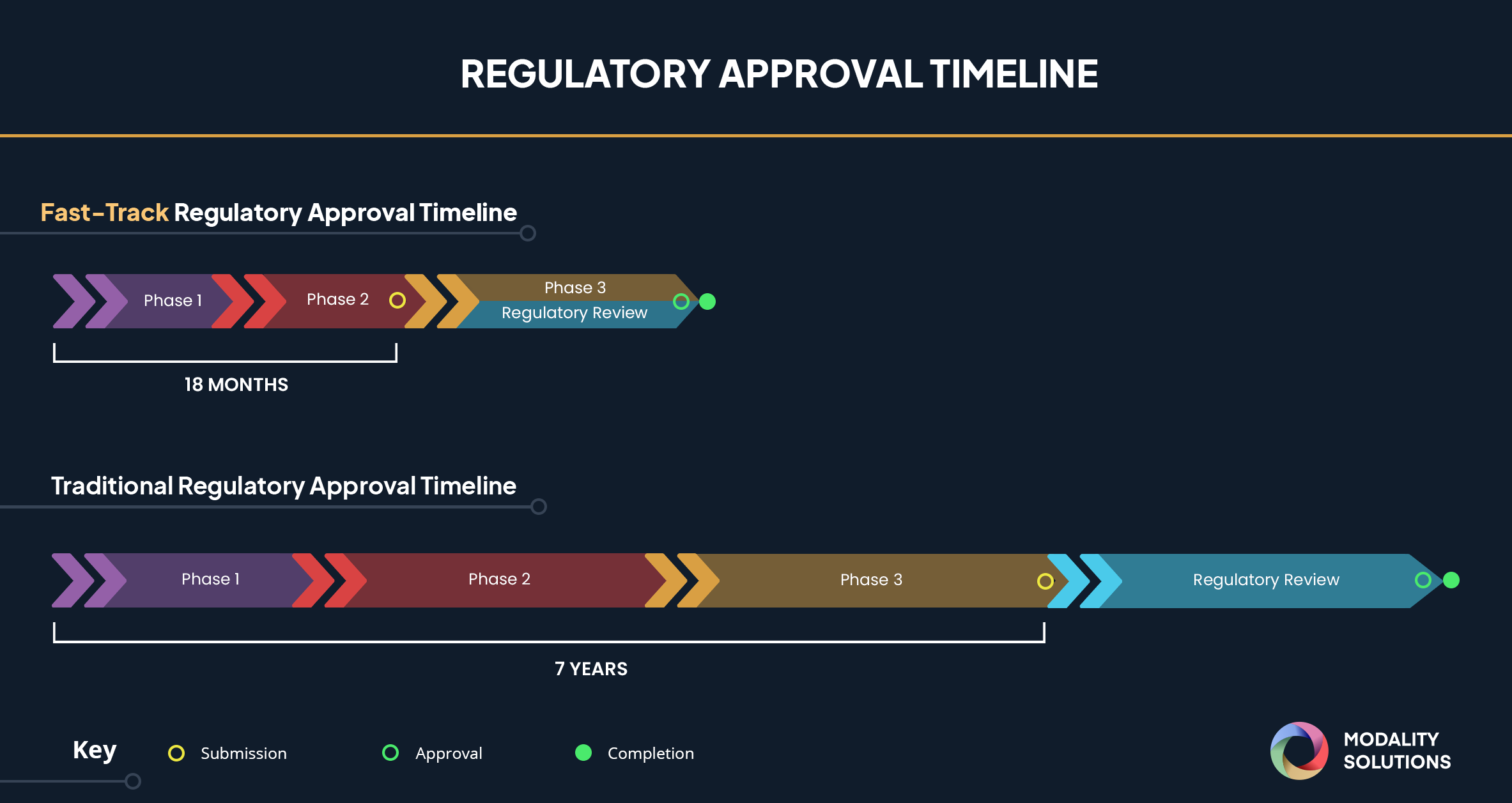
FDA's Fast Track Approval Coronavirus Treatment Acceleration Program
Rexulti (Brexpiprazole): Side Effects, Use for Depression, and More

FDA-Approved Drugs to Treat Schizophrenia Journal of Psychosocial Nursing and Mental Health Services
Recomendado para você
-
Rexulti Full Prescribing Information, Dosage & Side Effects21 março 2025
-
 Rexulti side effects and how to avoid them - NiceRx21 março 2025
Rexulti side effects and how to avoid them - NiceRx21 março 2025 -
 dtw Research, Inc on X: Throughout 2019, Rexulti produces the largest volume of marketing materials🔍📈 #lundbeck #otsuka #rexulti #brexpiprazole #depression #majordepressivedisorder #MDD #centralnervoussystem #CNS #antidepressants #yearendreview21 março 2025
dtw Research, Inc on X: Throughout 2019, Rexulti produces the largest volume of marketing materials🔍📈 #lundbeck #otsuka #rexulti #brexpiprazole #depression #majordepressivedisorder #MDD #centralnervoussystem #CNS #antidepressants #yearendreview21 março 2025 -
 Rexulti vs Abilify: Which is best for you?21 março 2025
Rexulti vs Abilify: Which is best for you?21 março 2025 -
 Rexulti, the first drug to relieve Alzheimer's emotions - TimesKuwait21 março 2025
Rexulti, the first drug to relieve Alzheimer's emotions - TimesKuwait21 março 2025 -
 Rexulti Patient Site - Once Daily Pharma21 março 2025
Rexulti Patient Site - Once Daily Pharma21 março 2025 -
 Pharmaceutical Business Products21 março 2025
Pharmaceutical Business Products21 março 2025 -
 REXULTI 0,5MG CAIXA COM 30 COMPRIMIDOS REVESTIDOS (C1) - Farmácias CallFarma21 março 2025
REXULTI 0,5MG CAIXA COM 30 COMPRIMIDOS REVESTIDOS (C1) - Farmácias CallFarma21 março 2025 -
 Rexulti 3 Mg, Antipsychotic - 28 Tablets21 março 2025
Rexulti 3 Mg, Antipsychotic - 28 Tablets21 março 2025 -
Rexulti Images Pill identification, Size, Shape and Color - BuzzRx21 março 2025
você pode gostar
-
 Kawaii Cute Cat Face with Ears. Positive Emotions. Cartoon Vector21 março 2025
Kawaii Cute Cat Face with Ears. Positive Emotions. Cartoon Vector21 março 2025 -
 BRUH LET ME LIVE : r/SlapBattles21 março 2025
BRUH LET ME LIVE : r/SlapBattles21 março 2025 -
 Uh, the LEGO Batman Movie seems good (98% RT)21 março 2025
Uh, the LEGO Batman Movie seems good (98% RT)21 março 2025 -
 Error occurred while logging into minecraft · Issue #1473 · gorilla-devs/GDLauncher · GitHub21 março 2025
Error occurred while logging into minecraft · Issue #1473 · gorilla-devs/GDLauncher · GitHub21 março 2025 -
 Tale Of Us reveals lineup for Afterlife Zamna Tulum 202221 março 2025
Tale Of Us reveals lineup for Afterlife Zamna Tulum 202221 março 2025 -
 Símbolos coloridos do teatro. conjunto de ícones de vetor de balé21 março 2025
Símbolos coloridos do teatro. conjunto de ícones de vetor de balé21 março 2025 -
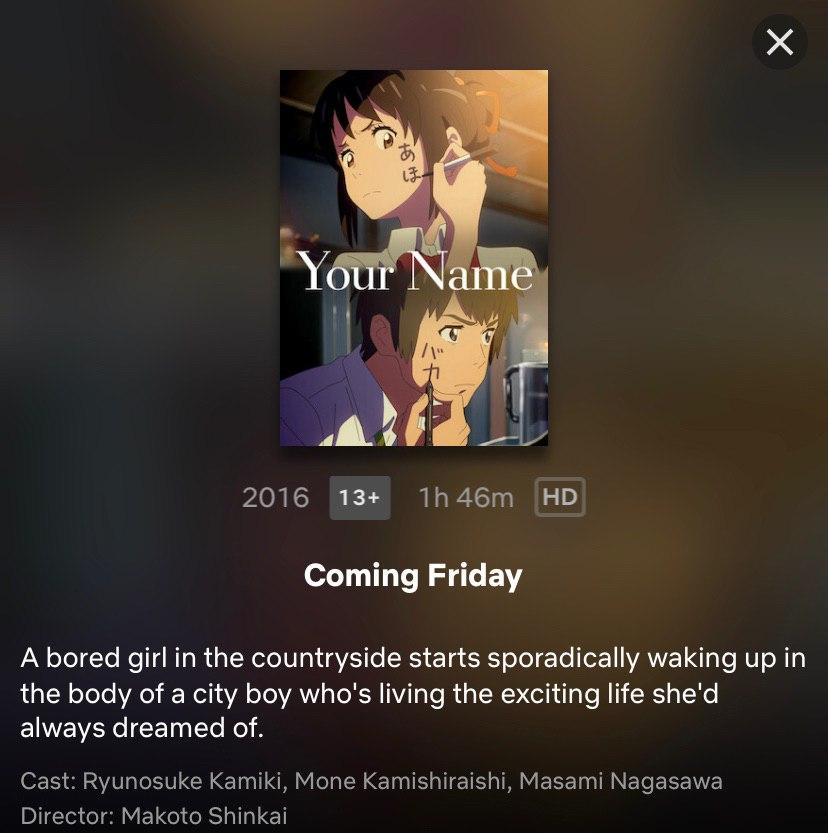 Your Name To Be Released On Netflix Philippines On July 10, 202021 março 2025
Your Name To Be Released On Netflix Philippines On July 10, 202021 março 2025 -
 Dealers Choice: How To Play Russian Roulette Poker21 março 2025
Dealers Choice: How To Play Russian Roulette Poker21 março 2025 -
 Ready player 4: Now I have 4 Bluetooth controllers - Steam couch21 março 2025
Ready player 4: Now I have 4 Bluetooth controllers - Steam couch21 março 2025 -
Palmeiras Social App - Microsoft Apps21 março 2025

