Hep B biotech Antios closed after FDA hold proved insurmountable
Por um escritor misterioso
Last updated 31 março 2025

Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable. | Viral disease biotech Antios Therapeutics shut down earlier this year after an FDA hold on its lead hepatitis B therapy due to a serious adverse event proved insurmountable.

Hepatitis B drug developers chart slow progress, just like in hep C

LinkedIn Landon Loving 페이지: Biotech pipeline hosts 163
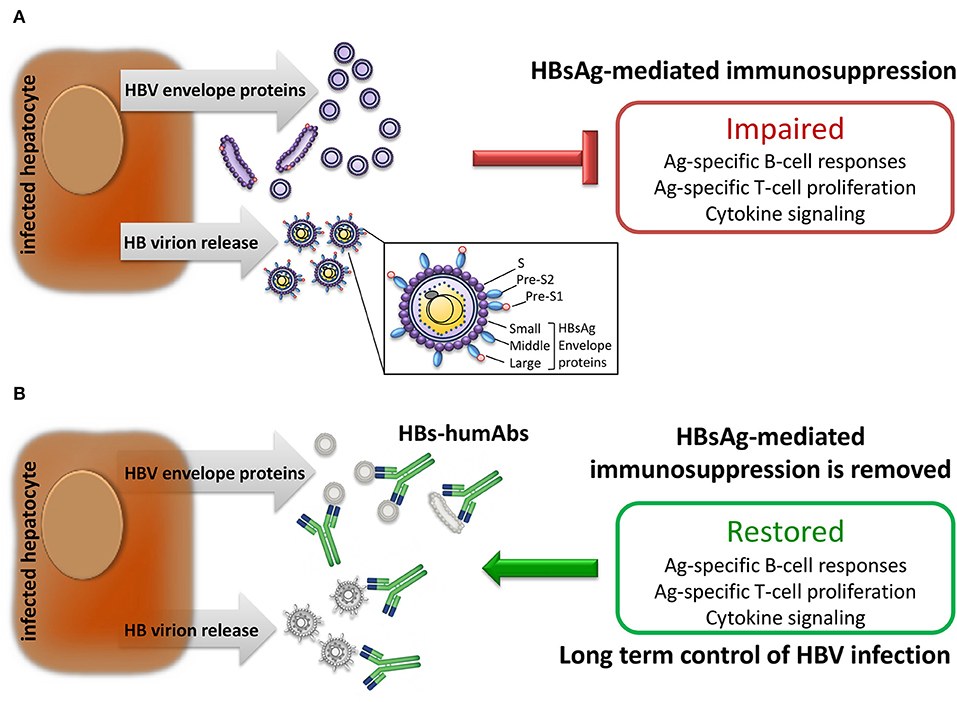
Frontiers Human Monoclonal Antibodies as Adjuvant Treatment of

Bone Scaffolds: An Incorporation of Biomaterials, Cells, and

Press Release: Novartis suspends US marketing and sales of Zelnorm

Hepatitis B drug developers chart slow progress, just like in hep C
33rd Annual Meeting & Pre-Conference Programs of the Society for

Double whammy sours mood at Antios Therapeutics
Biotechs jockey for gene therapy lead with hemophilia data

Annalee Armstrong - Journalist Profile - Intelligent Relations

Assembly Bio breaks off hepatitis B deal with Antios Therapeutics

Can Medivation's CEO get a bidding war started?
33rd Annual Meeting & Pre-Conference Programs of the Society for

Annalee Armstrong - Journalist Profile - Intelligent Relations

IHEP (International Hepatology Education Program)
Recomendado para você
-
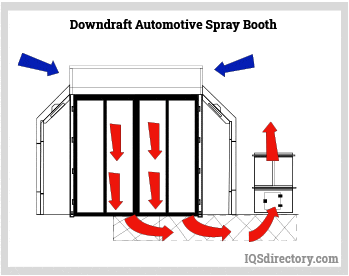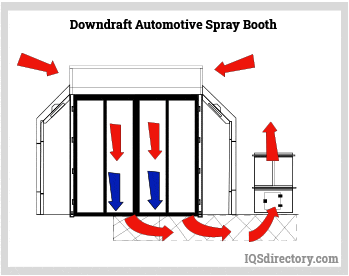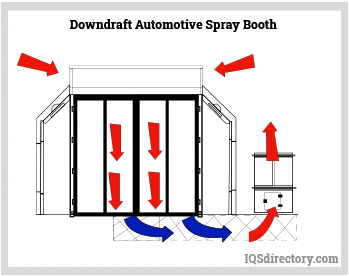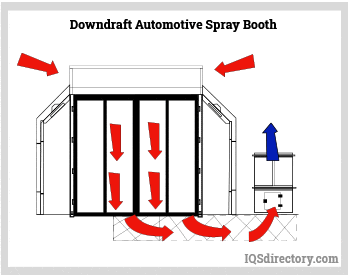 Paint Spray Booths: Construction, Types, Applications, and Benefits31 março 2025
Paint Spray Booths: Construction, Types, Applications, and Benefits31 março 2025 -
 Life in a Small Town: {State of the Kitchen} Part 5: Screeching Halt31 março 2025
Life in a Small Town: {State of the Kitchen} Part 5: Screeching Halt31 março 2025 -
 How Fortifi Financing Can Help You Upgrade to Impact Windows and Doors31 março 2025
How Fortifi Financing Can Help You Upgrade to Impact Windows and Doors31 março 2025 -
 Woa Is that a city you're building there? Dev Update #3 news - Cepheus Protocol - Mod DB31 março 2025
Woa Is that a city you're building there? Dev Update #3 news - Cepheus Protocol - Mod DB31 março 2025 -
 The Bern Convention Bureau Calls upon the Armenian Government to Halt Construction of the Gold Mine on Mt. Amulsar and Revise the ESIA31 março 2025
The Bern Convention Bureau Calls upon the Armenian Government to Halt Construction of the Gold Mine on Mt. Amulsar and Revise the ESIA31 março 2025 -
 Coronation of Charles III: an irresistible spectacle31 março 2025
Coronation of Charles III: an irresistible spectacle31 março 2025 -
 Knowledge Enterprise31 março 2025
Knowledge Enterprise31 março 2025 -
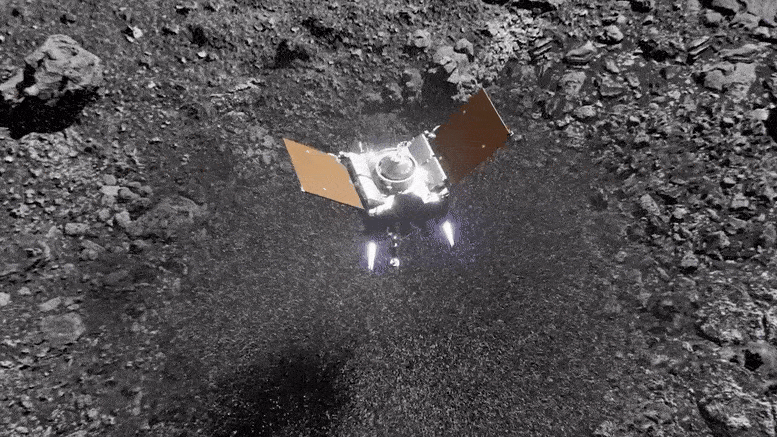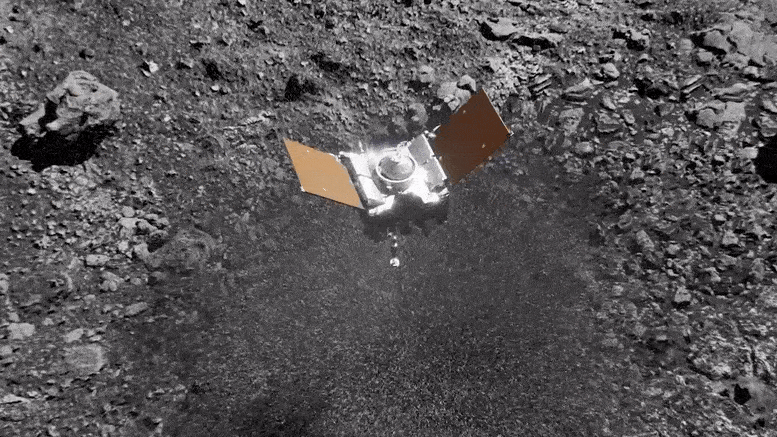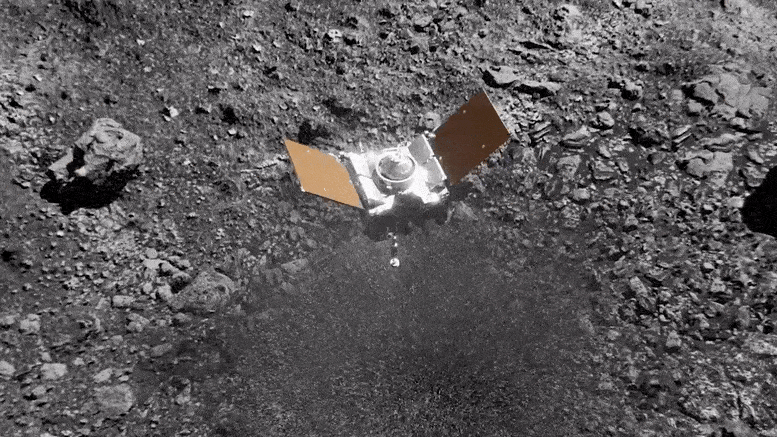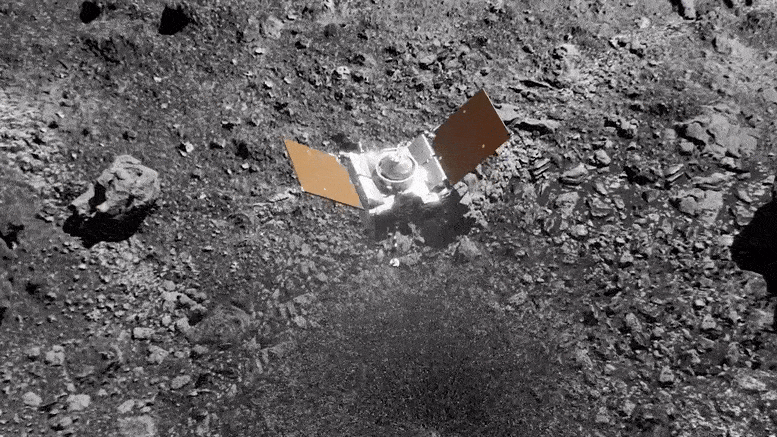 Preparing for Asteroid Bennu: NASA's OSIRIS-REx Astromaterials Lab Opens Doors to Media31 março 2025
Preparing for Asteroid Bennu: NASA's OSIRIS-REx Astromaterials Lab Opens Doors to Media31 março 2025 -
 BAKA artist on Game Jolt: I draw halt from roblox doors I hope31 março 2025
BAKA artist on Game Jolt: I draw halt from roblox doors I hope31 março 2025 -
 NTSB wants halt on doors-off sightseeing helicopter rides, 2019-12-1131 março 2025
NTSB wants halt on doors-off sightseeing helicopter rides, 2019-12-1131 março 2025
você pode gostar
-
 LC EP1: TP Link ER-605/ER-7206 , OC-200, SG-2210P, EAP-235 Omada & SDN Out-of-Box Experience31 março 2025
LC EP1: TP Link ER-605/ER-7206 , OC-200, SG-2210P, EAP-235 Omada & SDN Out-of-Box Experience31 março 2025 -
 Alphabet Lore VS Korean Alphabet (A-Z)31 março 2025
Alphabet Lore VS Korean Alphabet (A-Z)31 março 2025 -
 1 Jogo De Tabuleiro Biblico Para Crianças Personalizado31 março 2025
1 Jogo De Tabuleiro Biblico Para Crianças Personalizado31 março 2025 -
 Corinthians conhece adversário de estreia na Copa do Brasil 202331 março 2025
Corinthians conhece adversário de estreia na Copa do Brasil 202331 março 2025 -
Beekeeper's Naturals Propolis Immune Support Throat Spray - 1.06 Fl Oz : Target31 março 2025
-
 Jogos de Maquilhagem 🕹️ Jogue no CrazyGames31 março 2025
Jogos de Maquilhagem 🕹️ Jogue no CrazyGames31 março 2025 -
 Chess Universe - APK Download for Android31 março 2025
Chess Universe - APK Download for Android31 março 2025 -
 NotSlickBOi31 março 2025
NotSlickBOi31 março 2025 -
 Niehime to Kemono no Ou (A Princesa Oferenda e o Rei das Feras31 março 2025
Niehime to Kemono no Ou (A Princesa Oferenda e o Rei das Feras31 março 2025 -
 Triciclo Motoca Infantil Pedal Tchuco Baby Patrol31 março 2025
Triciclo Motoca Infantil Pedal Tchuco Baby Patrol31 março 2025
